AUTHORS: Sharon A. Gutman, Kristin A. Gregory, Megan M. Sadlier-Brown, Marcy A. Schlissel, Allison M. Schubert, Lee Ann Westover, and Richard C. Miller.
Abstract
Although sleep intervention is within the domain of occupational therapy, few studies exist supporting practice. Effectiveness of three sleep interventions was compared: Dreampad Pillow®, iRest® meditation, and sleep hygiene. Twenty-nine participants were randomly assigned to the Dreampad Pillow® (n = 10), iRest® meditation (n = 9), and sleep hygiene (n =10) groups. In Phase 1, all participants used a 7-day sleep hygiene regimen to reduce poor sleep habits. In Phase 2 (14 days), 10 participants used the Dreampad Pillow® and sleep hygiene, nine used the iRest meditation and sleep hygiene, and 10 continued sleep hygiene only. At intervention-end, the iRest meditation group experienced statistically greater time asleep than both the Dreampad Pillow® (p < .006, d = 1.87) and sleep hygiene groups (p < .03, d = 1.80). The Dreampad Pillow® group experienced statistically fewer nighttime awakenings than the iRest® meditation (p < .04, d = −1.53) and sleep hygiene (p < .004, d = −1.43) groups. No differences were found between groups in perceived sleep quality, length of time needed to fall asleep, and fatigue level next day. This study provides support for sleep interventions within occupational therapy’s domain.
Fifty to 70 million Americans are affected by chronic sleep disorders that can significantly diminish health, alertness, and safety (Liu et al., 2013). In addition, one third of Americans get less than 7 hr of sleep (Schoenborn, Adams, & Peregoy, 2013), drowsy drivers may cause 5,000 to 6,000 fatal crashes each year (Watson et al., 2015), and 70% of high school students do not get the required amount of sleep on school nights (Mcknight-Eily et al., 2011). Daytime sleepiness is associated with elevated risk of heart disease and stroke (Gangwisch, 2014), and an estimated US$59.8 billion in productivity are lost each year due to disordered sleep (Kessler, 2011). Eighty-three percent of individuals who experience sleep disturbance self-treat with over-the- counter medications and dietary supplements. Once help is sought, one third of patients go directly to a sleep specialist, while the remainder turn to practitioners with whom they have an established relationship—most frequently a gynecologist or general practitioner (Henry, Rosenthal, Dedrick, & Taylor, 2013).
Currently, several health care practitioners—physicians, psychiatrists, and dentists—commonly treat sleep disorders using pharmacological interventions as first-line treatment agents. Despite the high use of sleep medication, increasing evidence has demonstrated that long-term use of such drugs may have negative side-effects including dependency, day- time drowsiness, nausea, fatigue, confusion, and memory problems (Ramar & Olsen, 2013). Because of the negative side-effects of pharmacological interventions, adults with dis- ordered sleep are increasingly seeking non-pharmacological sleep interventions such as natural supplements (e.g., melatonin, valerian, and tryptophan), meditation, and sleep hygiene programs (Henry et al., 2013).
In recent years, the American Occupational Therapy Association (AOTA, 2014) has called for occupational therapists to become involved in patient sleep hygiene problems as they affect daily function. Sleep hygiene is a set of behaviors that are believed to contribute to improved sleep. For example, the National Sleep Foundation (2016) recommends the fol- lowing to improve sleep quality: maintaining consistent sleep and wake schedules, reducing afternoon naps, avoiding bright light and TV/computer use before bed, and avoiding alcohol, caffeine, cigarettes, as well as food intake in the evening. Occupational therapists commonly treat patients with both physical and mental health disabilities that contribute to disordered sleep (e.g., pain syndromes resulting from musculoskel- etal problems, head injuries and neurological diseases disrupting circadian rhythms, and neurochemically based mental health problems that disrupt the neurotransmitters responsible for sleep onset and maintenance). Poor quality and loss of sleep can cause daytime drowsiness and fatigue that affect client performance level and motivation (Caruso, 2014). Hospital inpatients may be particularly vulnerable to poor sleep due to hospital noise and activity occurring throughout the night (Buxton et al., 2012).
Over the last years, the occupational therapy literature has increasingly addressed non-pharmacological sleep interventions to enhance clients’ sleep quality. Such practices include helping clients to select mattresses, wedges, and pillows that reduce neuromuscular pain (Fung, Wiseman-Hakes, Stergiou- Kita, Nguyen, & Colantonio, 2013); modifying the sleep envi- ronment through adjustment to lighting, noise level, and external distractions (Wooster et al., 2015); and helping clients create and maintain daily life and sleep hygiene routines that promote optimal sleep (Blanchard et al., 2015; Garms- Homolovà, Flick, & Rohnsch, 2010; Wooster et al., 2015). While the profession has begun to contribute to this practice area, few research studies have been published to date provid- ing support for the effectiveness of our sleep interventions.
The purpose of this study was to compare three non-pharmacological sleep interventions designed to increase sleep quality through environmental modification and internal regulation. One intervention was a pillow that produces audio vibrations conducted to the user’s inner ear (i.e., Dreampad Pillow®). The vibrations are transmitted through electromechanical transducers embedded in the pillow core and are audible only to the user. These vibrations are believed to increase parasympathetic nervous system activity that helps to slow physiological and cognitive processes (Olson, 2014); however, the neurophysiological mechanisms under- lying the pillow’s effect have not been examined. The Dreampad Pillow® has been shown to have some effectiveness improving sleep quality in 15 children with autism spectrum disorder (Schoen, 2014), eight children with attention deficit hyperactivity disorder (Hallowell Center, 2012), and
10 veterans with posttraumatic stress disorder (PTSD; Nelson, 2014). Although this initial effectiveness research is promising, the sample sizes in all three studies were small, and control and randomization were not used raising questions about internal and external validity.
A second intervention was a yogic meditation audio pro- gram produced by the Integrative Restoration Institute, or iRest® (Miller, 2015). The iRest® sleep meditation program is based on yoga nidra principles (i.e., practices that promote the state between sleep and wakefulness) and provides verbal narration to guide users to a restful state of body and mind (Miller, 2010). Although a number of studies have demonstrated that the iRest® meditation program has helped decrease stress levels in veterans with PTSD (Stankovic, 2011), female patients with multiple sclerosis and cancer (Pritchard, Elison- Bowers, & Birdsall, 2010), students with anxiety (Eastman- Mueller, Wilson, Jung, Kimura, & Tarrant, 2013), adults with chemical dependence (Temme, Fenster, & Ream, 2012), veterans with chronic pain (Nassif et al., 2016), and health care providers (Bingham, Inman, Walter, Zhang, & Peacock, 2012), no studies to date have assessed the iRest® meditation pro- gram’s ability to improve sleep quality.
A third intervention consisted of five sleep hygiene prac- tices recommended by the National Sleep Foundation (2016; e.g., avoiding food intake, alcohol, caffeine, and screen time before bed). Although these three interventions were not developed by occupational therapists, they fall within our domain of practice and address internal regulation and environmental modification to enhance sleep. We first sought to determine if the 1-week sleep hygiene protocol made a statistically significant difference in sleep quality when compared with baseline data. Our second research question asked whether a statistically significant difference existed between all three intervention groups on five sleep measures at intervention-end: length of time needed to fall asleep, total time asleep, number of nighttime awakenings, length of nighttime awakenings, and fatigue level next day.
Method
Research Design
The research design was a comparison of the effectiveness of three sleep interventions. Once participants were recruited from a convenience sample, they were randomly assigned to either Dreampad Pillow®, iRest® meditation, or sleep hygiene control groups. All participants were asked to adhere to a 1-week sleep hygiene protocol after baseline data collection and before the 2-week Dreampad Pillow® and iRest® meditation interventions commenced. We hoped that a 1-week sleep hygiene period would eliminate poor sleep behaviors that could affect the effectiveness of the Dreampad Pillow® and iRest® meditation program. Upon initiation of the Dreampad Pillow® and iRest® meditation interventions, one participant group continued using the sleep hygiene protocol alone and served as a control. This study was approved by Columbia University’s institutional review board (IRB), and all participants provided consent.
Participants
Participants were recruited by word of mouth. Once a participant was recruited, he or she was asked to identify other potential participants who then contacted the researchers for further information. To be included in the study, participants had to be between 25 and 65 years of age, self-report poor sleep for at least 2 months, and be willing to adhere to a sleep hygiene protocol for 3 consecutive weeks. Participants were excluded if they were currently taking a prescribed or over- the-counter sleep medication, currently taking medication causing drowsiness or alertness, had neck or back pain preventing use of the Dreampad Pillow®, commonly experienced motion sickness (sometimes reported from Dreampad Pillow® use), had a medical diagnosis causing sleep disruption, had pets or family members causing sleep disruption, were pregnant, or were smokers (see Figure 1).
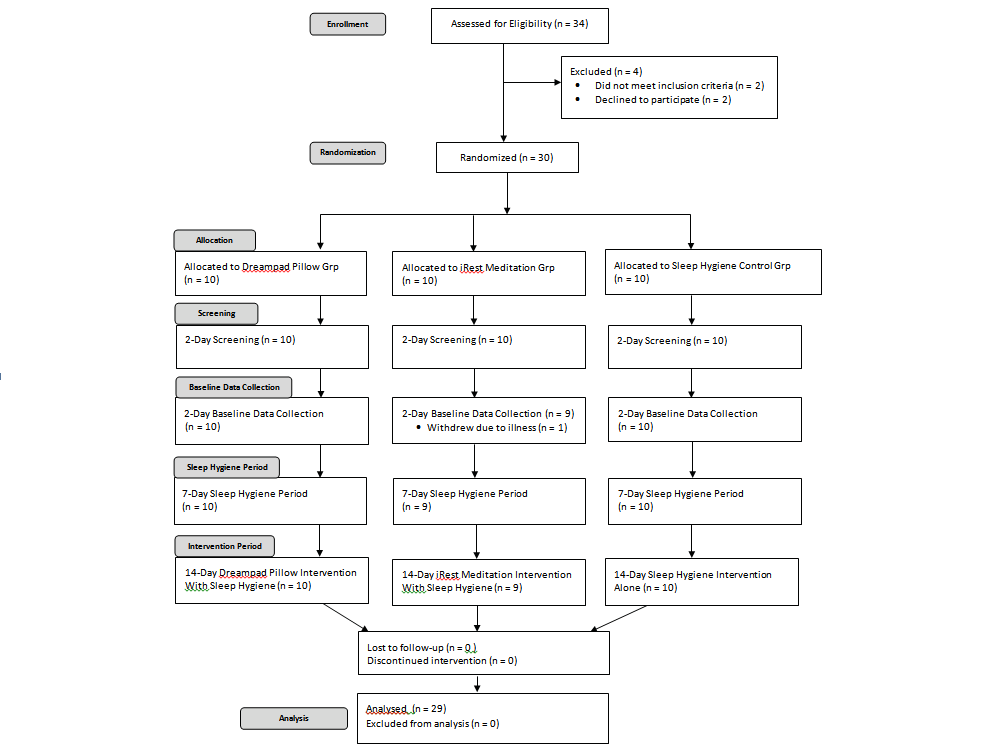
Figure 1. Flowchart illustrating participant recruitment, enrollment, group allocation, screening, baseline data collection, sleep hygiene period, intervention period, and analysis.
Instruments and Outcome Measures
General Sleep Disturbance Scale (GSDS). The GSDS is a 21-item, self-report Likert-type scale developed to identify and assess perceived sleep quality, sleep disturbance
frequency, sleep initiation, early wakening, daytime mood, fatigue level, and sleep aid use (Lee, 2012). The GSDS requires 5 to 10 min to complete. Total GSDS scores can range from 0 (no disturbance) to 147 (extreme sleep disturbance). Each mean subscore can range from 0 to 7. Higher total and subscale scores indicate greater levels of sleep disturbance. Subscales scores ³3 and a GSDS total score ³43 indicate significant sleep disturbance. Internal consistency of the scale was found to be high with a Cronbach’s α of .88 (Lee, 1992). The GSDS was administered before baseline data collection and again at post-intervention (2 days after intervention completion).
Pittsburgh Sleep Quality Index (PSQI). The PSQI is a self- report measure designed to assess the perceived sleep qual- ity and sleep patterns in adults (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). This assessment requires 10 min to complete and contains 19 questions that address seven main areas as experienced by the respondent in the last 30 days: sleep efficiency, sleep difficulties, sleep length, sleep quality, sleep disruptions, sleep medication use, and day- time drowsiness. The first four questions require exact numerical answers based on number of hours slept, time sleep was initiated, and time respondent awoke. Questions 5 to 9 are based on a Likert-type scale of 0 to 3, where 3 denotes higher levels of sleep difficulty. The final scoring system to calculate whether the respondent has disordered sleep consists of seven components with a global score ranging from 0 to 21. A total global score of ³5 demonstrates that the respondent experiences poor sleep. Internal consistency has been established as high with a Cronbach’s α of .83 for its seven main factors (Smyth, 2012); a test– retest correlation coefficient of .85 has also been established (Choi, Kim, Beomjong, & Kim, 2015). The PSQI was administered before baseline data collection and again at post-intervention (2 days after intervention completion).
Actigraph accelerometer. The Jawbone® actigraph wristband is an accelerometer that measures the length of time required to fall asleep, total time asleep, and number and length of nighttime awakenings. The Jawbone® actigraph demonstrated moderate reliability (r = .74, p < .01) when compared with seven consumer accelerometers (e.g., Bodymedia Fit®, Fitbit Zip®, Nike Fuel Band®; Lee, Kim, & Welk, 2014). Mean absolute percent error was found to be 12.2%. The actigraph was worn by participants during the 2-day baseline data collection, 1-week sleep hygiene period, and 2-week intervention period.
Sleep journal. Participants maintained a daily sleep journal in which they were asked to record information about the quantity and quality of their sleep. The sleep journal was recorded in hard copy format, and email reminders were sent every second day to help participants remember to complete daily sleep journal entries. Participants recorded sleep journal entries during the 2-day baseline data collection period, 1-week sleep hygiene period, and 2-week inter- vention period. Participants responded to the following questions: (a) What time did you go to bed? (b) Did you have difficulty falling asleep? (c) How long did it take you to fall asleep? (d) Did you wake during the night? (e) How many times do you remember waking during the night? (f) How long did your awakenings last before you fell back to sleep? (g) What time did you awake in the morning? (h) How many hours do you estimate having slept? (i) On a scale from 1 to 5 (1 = very tired, 5 = very alert), how tired or alert did you feel the next day?
Procedures
Assignment to intervention groups. After enrollment, all participants were randomly assigned to the Dreampad Pillow®, iRest® meditation, or sleep hygiene control group using a random numbers generator.
Screening. After group assignment, all participants were educated in the use of their assigned intervention and actigraph wristband. Participants were asked to use these devices for a 2-day period in their homes to determine whether they could tolerate wearing the actigraph wristband during sleep and whether the Dreampad Pillow® group members could toler- ate using the pillow without experiencing motion sickness. Although we planned to terminate and replace participants who could not tolerate these devices, no problems were reported. Participants were also instructed in use of the sleep hygiene protocol that included (a) alcohol restriction (no more than one drink per day and no alcohol 2 hr before bed- time), (b) caffeine restriction (no caffeine after 2:00 p.m.), (c) food intake restriction (no food after 7:00 p.m.), (d) screen time restriction (no television, Internet, computer, or tablet 1 hr before bedtime), and (e) no daytime naps longer than 30 min. These sleep hygiene behaviors are recommended by the National Sleep Foundation (2016). Participants also completed the pre-intervention GSDS and PSQI during screening (see Figure 2). All participants were provided with contact information for the research assistants to trouble-shoot technology problems or answer study-related questions.
Baseline data collection. Directly following the 2-day screening period, all participants were asked to use their actigraph wristbands and sleep journals to record sleep information for two consecutive nights. During baseline data collection, participants did not use their assigned interventions or sleep hygiene protocol (see Figure 2).
Sleep hygiene period. Directly following the 2-day baseline data collection period, the 7-day sleep hygiene period occurred. In this study phase, participants were asked to adhere to the sleep hygiene protocol and use the actigraph wristband and sleep journal to record sleep information (see Figure 2). If problems or concerns arose, participants were encouraged to contact a research assistant. Email reminders were sent to participants every second day to remind them to complete their sleep journal entries and report problems adhering to the sleep hygiene protocol. The sleep hygiene period was used to help participants eliminate poor sleep behaviors that could affect the effectiveness of the Dreampad Pillow® and iRest® meditation interventions. We also used the sleep hygiene period to determine if these commonly rec- ommended sleep behaviors improved sleep quality (i.e., time needed to fall asleep, total time asleep, number of nighttime awakenings, length of nighttime awakenings, and fatigue level next day) as compared with baseline data.
Intervention period. At the end of the sleep hygiene period, the 14-day intervention period commenced. In this study phase, participants assigned to the Dreampad Pillow® and iRest® meditation groups began to use these interventions while continuing the sleep hygiene protocol. Participants assigned to the sleep hygiene control group continued using the sleep hygiene protocol alone. We hoped that this design would allow us to determine if the Dreampad Pillow® and iRest® meditation program had additive effects above and beyond the sleep hygiene protocol. At bedtime, participants programmed the Dreampad Pillow® to run for 2 hr, after which time it automatically turned off. iRest® meditation participants used the program for 20 min before bed. During the intervention period, participants continued to contact the research assistants for help with technology questions. Email reminders continued to be sent every second day to remind participants to complete their daily sleep journal entries and report problems adhering to the sleep hygiene protocol.
Two-day post-intervention period. Two days after intervention completion, participants completed the post GSDS and PSQI.
Data Collection

Figure 2. Flowchart illustrating the sequence of outcome
administration.
Note. GSDS = General Sleep Disturbance Scale; PSQI = Pittsburgh sleep quality index.
The pre-intervention GSDS and PSQI, both self-report measures, were administered as pre-intervention assessments by a research assistant during screening. The sleep journals and actigraph wristbands were used to record data during the 2-day baseline period, 7-day sleep hygiene period, and 14-day intervention period. The post-intervention GSDS and PSQI were completed by participants 2 days after interven- tion-end and emailed or mailed to the researchers. Sleep journal data were also emailed or mailed to the researchers at study completion. Actigraph wristband data were automatically and continuously uploaded to and stored on a pass- word-protected server that could only be accessed by the researchers (see Figure 2).
Data Analysis
To determine whether all three groups were equivalent on baseline measures, we used a Kruskal–Wallis test for the GSDS and PSQI, and an ANOVA for four parametric-level sleep measures recorded by the sleep journal and actigraph wristband (i.e., length of time to fall asleep, number and length of nighttime awakenings, and total time asleep). For the non–parametric-level sleep measure, fatigue level next day, we used a Kruskal–Wallis test to determine if all groups were equivalent at baseline (Portney & Watkins, 2015).
To determine if a statistically significant difference existed between groups on the four parametric sleep mea- sures after adhering to the 1-week sleep hygiene regimen, we used an ANOVA with a post hoc Tukey analysis. The non- parametric sleep measure, fatigue level next day, was ana- lyzed using a Kruskal–Wallis test and a Mann–Whitney U post hoc analysis to identify between-group differences (Portney & Watkins, 2015).
To determine if a statistically significant difference existed between all three groups at post-intervention on the GSDS and PSQI, we used a Kruskal–Wallis test with a Mann–Whitney U post hoc analysis. An ANOVA with a post hoc Tukey analysis was used to determine if a statistically significant change was observed between groups at interven- tion end for the four parametric-level sleep measures. The fifth sleep measure, fatigue level next day, was analyzed with a Kruskal–Wallis test and Mann–Whitney U post hoc analy- sis (Portney & Watkins, 2015).
Data were analyzed using SPSS Version 23, and significance level was set at α < .05. A power analysis suggested that with a sample size of 10 subjects per group and a standard deviation of 1 hr of sleep, we were powered to detect between-group differences of 1.3 hr.
Results
Thirty adults with self-reported disordered sleep were enrolled in the study (see Figure 1). One participant with- drew during baseline data collection because of injury; 29 participants (female = 20, male = 9) completed the study. Participant ages ranged from 25 to 65 years (M = 43.24, SD = 12.26 years). Race and ethnicity included White (n = 24, 82.75%), African American (n = 2, 6.89%), Asian (n = 2, 6.89%), and Hispanic (n = 1, 3.44%). Nine participants (31.03%) completed college and 20 (68.96%) completed graduate education. Reported participant sleep problems included difficulty falling asleep (n = 8, 27.58%), nighttime awakening (n = 29, 100%), and difficulty falling back to sleep once awakened (n = 16, 55.17%).
Analysis of baseline data using an ANOVA for parametric data and a Kruskal–Wallis test for nonparametric data showed that all groups were equivalent on all sleep measures (i.e., time needed to fall asleep, number and length of night- time awakenings, total time asleep, fatigue level next day). Participants reported that they slept an average of 6 hr and 38 min per night (SD = 1.06 hr), required an average of 23 min to fall asleep (SD = 3.55 min), experienced at least two night- time awakenings (SD = 0.05) lasting an average of 20 min each (SD = 7.74 min), and felt “somewhat tired” the next day (SD = 0.55).
At the end of the 1-week sleep hygiene period, no statisti- cally significant differences were found between and within groups on all sleep measures suggesting that the sleep hygiene protocol did not affect sleep problems identified at baseline.
At intervention-end (the final 2 days of Phase 2), no sta- tistically significant differences were found between groups on the following primary outcome measures: length of time needed to fall asleep, length of nighttime awakenings, and fatigue level next day.
Statistically significant differences were found at inter- vention-end between groups on two primary outcome sleep measures: total time asleep and number of nighttime awak- enings. Using the participants’ sleep journal, an ANOVA found a statistically significant difference between all three groups on length of time asleep, F(2, 28) = 6.343, p < .006. A post hoc Tukey analysis found a statistically significant difference between the iRest® meditation and the Dreampad Pillow® groups (p < .006), with a large effect size (Cohen’s d = 1.87). A statistically significant difference was also found between the iRest® meditation and the sleep hygiene control groups (p < .03), with a large effect size (Cohen’s d = 1.80). These results were confirmed by the actigraph data that also
demonstrated a statistically significant difference between groups on length of time asleep, F(2, 28) = 5.16, p < .01. A post hoc Tukey analysis of the actigraph data confirmed the statistically significant difference found between the iRest® meditation and the Dreampad Pillow® groups (p < .02, Cohen’s d = 1.29). The post hoc Tukey analysis also con- firmed the statistically significant difference found between the iRest® meditation and the sleep hygiene control groups (p < .03, Cohen’s d = 2.37). These data suggest that at inter- vention-end, the iRest® meditation group experienced a sta- tistically significant greater time asleep than both the Dreampad Pillow® and sleep hygiene control groups (see Table 1).
Using sleep journal data, an ANOVA identified a statisti- cally significant difference between groups on number of nighttime awakenings, F(2, 28) = 6.97, p < .004. A post hoc Tukey analysis found a statistically significant difference between the Dreampad Pillow® and iRest® meditation groups (p < .04, Cohen’s d = −1.53), and the Dreampad Pillow® and sleep hygiene control groups (p < .004, Cohen’s d = −1.43). These results were confirmed by analysis of the actigraph data, which also demonstrated a statistically sig- nificant difference in number of nighttime awakenings between groups, F(2, 28) = 4.90, p < .02. Post hoc Tukey analysis of the actigraph data confirmed the statistically sig- nificant difference found between both the Dreampad Pillow® and iRest® meditation groups (p < .02, Cohen’s d =−1.12), and the Dreampad Pillow® and sleep hygiene con- trol groups (p < .04, Cohen’s d = −1.47). These data suggest that at intervention-end, the Dreampad Pillow® group expe- rienced a statistically significant fewer amount of nighttime awakenings than both the iRest® meditation and sleep hygiene control groups (see Table 1).
Although at intervention-end, the iRest® meditation group participants experienced greater time asleep, and the Dreampad Pillow® group participants experienced fewer nighttime awakenings, these changes were not sufficient to change the participants’ perceived sleep quality—a study secondary outcome measure. No statistically significant dif- ference was found between and within groups at interven- tion-end on both the GSDS and PSQI.
Discussion
The purpose of this study was to compare the effects of three sleep interventions with otherwise healthy adults who self- reported disordered sleep. In Phase 1 of the study, we asked all participants to adhere to a 7-day sleep hygiene protocol to eliminate the behaviors that are believed to contribute to sleep problems (National Sleep Foundation, 2016). That our study found no statistically significant difference between the baseline and sleep hygiene period is not dissimilar to the mixed body of sleep hygiene literature. Although caffeine and alcohol intake have been highly correlated with sleep problems (Roehrs & Roth, 2001, 2008), many other typically recommended sleep hygiene behaviors (e.g., limiting exercise, reading, Internet, and television viewing before bed) have not been shown to strongly correlate with improved sleep quality (Custers & Van den Bulck, 2012). Based on our findings and that of the literature, it is unclear whether sleep hygiene behaviors other than limiting caffeine, alcohol, and food intake in the evening and maintaining consistent sleep and wake times, should be recommended to patients.
Table 1. Comparison of Dreampad Pillow®, iRest®, and Sleep Hygiene Groups at Post-Intervention.
|
GSDS |
PSQI |
Total time asleep (min) | No. of nighttime awakenings | Length of nighttime awakenings (min) | Length of time to fall asleep (min) | Fatigue level next day |
| Dreampad M = 28.36 | M = 16.93 | Sleep Journal | *Sleep Journal | Sleep Journal | Sleep Journal | M = 2.4 |
| Pillow® SD = 5.57 | SD = 3.72 | M = 358.35
SD = 62.16 |
M = 1.07
SD = 0.86 |
M = 40.23
SD = 10.29 |
M = 16.55
SD = 7.97 |
SD = 0.45 |
| Actigraph
M = 377.35 SD = 70.77 |
*Actigraph
M = 1.1 SD = 0.77 |
Actigraph
M = 42.10 SD = 16.08 |
Actigraph
M = 18.25 SD = 9.85 |
|||
| iRest® M = 31.42 | M = 14.46 | *Sleep Journal | Sleep Journal | Sleep Journal | Sleep Journal | M = 2.3 |
| SD = 5.88 | SD = 5.76 | M = 459
SD = 43.7 |
M = 1.92
SD = 0.85 |
M = 39.42
SD = 12.84 |
M = 14.63
SD = 6.89 |
SD = 0.63 |
| *Actigraph
M = 455.95 SD = 48.98 |
Actigraph
M = 2.35 SD = 1.37 |
Actigraph
M = 36.35 SD = 13.92 |
Actigraph
M = 15.50 SD = 7.46 |
|||
| Sleep Hygiene M = 29.27 | M = 15.38 | Sleep Journal | Sleep Journal | Sleep Journal | Sleep Journal | M = 2.3 |
| SD = 6.18 | SD = 4.81 | M = 361.74
SD = 62.46 |
M = 2.10
SD = 1.13 |
M = 41.59
SD = 15.71 |
M = 16.50
SD = 6.37 |
SD = 0.47 |
| Actigraph
M = 379.05 SD = 46.15 |
Actigraph
M = 2.30 SD = 0.85 |
Actigraph
M = 45.70 SD = 18.05 |
Actigraph
M = 19.95 SD = 8.37 |
Note. GSDS = General Sleep Disturbance Scale; PSQI = Pittsburgh sleep quality index.
*Indicates statistical significance at p < .05 or below.
Our study found two statistically significant changes in sleep quality at intervention-end: (a) the iRest® meditation group experienced greater total sleep time than both the Dreampad Pillow® and the sleep hygiene control groups, and (b) the Dreampad Pillow® group experienced fewer nighttime awakenings than both the iRest® meditation and sleep hygiene control groups. Length of nighttime awaken- ings, however, was not affected. Based on these findings, we recommend an integrated use of the iRest® meditation and Dreampad Pillow® interventions to increase total sleep time and reduce nighttime awakenings. Perhaps the Dreampad Pillow® manufacturers could develop an option to use a guided sleep meditation with ambient music during and fol- lowing the meditation.
Our study did not address intervention dosage, but dosage could greatly affect the effectiveness of both interventions. As recommended by the Dreampad Pillow® manufacturers (Integrated Listening Systems, n.d.), we asked participants to program the ambient music to turn off automatically after 2 hr. Dreampad Pillow® settings, however, can be programmed to allow the ambient music to play for extended hours or all night. Future research should discern whether extended hours of ambient music further reduce nighttime awakenings. Extended
hours of ambient music may also assist participants to fall back to sleep if they awaken. We recommend, too, the development of an ergonomically designed, memory foam Dreampad Pillow® that better fits the natural curves of the head and neck. This pillow design is particularly important for people with vertebral and upper extremity musculoskeletal problems.
In our study, dosage of the iRest® meditation program was similarly unassessed. The iRest® meditation creators suggest that participants may benefit from a daytime stress reduction meditation program as well as one used before sleep (Miller, personal communication, September 15, 2015). Miller proposes that a daytime meditation program may have additive effects to the sleep meditation program— with more practice, people may become better skilled at releasing stressful thoughts that prevent sleep. Using the iRest® meditation program during nighttime awakenings may also help people to fall back to sleep more quickly. These varying forms of iRest® meditation use and dosage should be assessed in future studies to best understand the potential effectiveness of this sleep intervention.
Despite the iRest® meditation group’s increased total time asleep at intervention-end and the Dreampad Pillow® group’s decreased nighttime awakenings, these sleep changes were not sufficient to alter the participants’ perceived sleep quality as measured by the GSDS and PSQI. Perhaps to per- ceive improved sleep quality, participants needed to experi- ence improvement in two or more sleep measures. Or perhaps participants needed to experience a change in daytime fatigue level. Factors that influence the perception of sleep quality should be further explored to better understand how to develop effective interventions.
Limitations
Although our sample size was small (N = 29), with a standard deviation of 1 hr of sleep and 10 participants per group, we were powered to detect between-group differences of 1.3 hr. Nevertheless, a study with a larger size sample would yield higher powered results. One limitation of our sample was that it was derived from a healthy adult population who reported disordered sleep but had no other health problems. It is unclear whether the data derived from this sample would be useful to populations of adults with mental health and physical disabili- ties. Although it is clear that poor sleep can exacerbate mental health and physical disabilities (Buxton et al., 2012; Caruso, 2014), the findings in this study cannot be generalized to popu- lations with formal acute and chronic health problems. Both the Dreampad Pillow® and iRest® meditation programs must be assessed with adults with varying disabilities.
Another limitation of this study concerned the use of a self- report sleep journal and actigraph wristband. Self-report instruments—particularly those requiring people to remember sleep information occurring the night before—may be inac- curate. Similarly, the actigraph accelerometer that we used had moderate reliability (r = .74, p < .01) and a mean absolute percent error of 12.2% when compared with seven consumer accelerometers (Lee et al., 2014). Although both the self- report journal and the actigraph wristband may have had some degree of inaccuracy, each supported the findings of the other, lending greater credibility to both instruments in this study.
A final limitation of this study related to the possible influence of providing sleep hygiene instructions to participants during screening. Although information about sleep hygiene practices was provided to participants 4 days before the sleep hygiene period, participants reported that they did not alter their behaviors until the first day of the 1-week sleep hygiene period.
Future Research
We suggest that both the iRest® meditation and Dreampad Pillow® interventions be integrated and assessed with populations reporting disordered sleep and who have mental health and physical disabilities. Future testing should also involve an assess- ment of varying dosages. Although this study addressed adults, future testing should involve children with sleep disorders.
Conclusion
Occupational therapists work with acute and chronic populations with mental health and physical disabilities. Because the link between poor sleep and daily function is clear (Kessler, 2011), and because occupational therapists help patients to function optimally in daily living activities despite disability, it is critical that we build our scope of practice to address patient sleep problems. This study contributes to the growing body of research providing support for sleep interventions within the domain of occupational therapy. Modification of
the sleep environment using audio pillows such as the Dreampad Pillow® and helping people to internally regulate and reduce stress through sleep meditation programs such as iRest® are two interventions that have been shown to positively affect sleep quality. Further research addressing sleep environment modification, internal regulation, sleep hygiene behaviors, and daily activity levels is needed to support the profession’s role in this practice area.
Authors’ Note
This study was approved by Columbia University Medical Center’s IRB (AAAP8360), and all participants provided informed consent.
Acknowledgments
The authors thank Randall Redfield, CEO, Integrated Listening Systems®, for donating equipment and Trisha Barry for iRest® mediation instruction.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, author- ship, and/or publication of this article.
